By Heather Koyle, Elisa Cottam,
and Geneva Chamblee
Movie demo beneath picture
By Heather Koyle, Elisa Cottam,
and Geneva Chamblee
Movie demo beneath picture

Materials:
 lab
coat
lab
coat
Henry Cavendish 1766
1766- Historical event-American anger brings repel of stamp acts
1766- Cultural event-Oldest church surviving in Manhattan was built as a subsidary to Trinity church
1766- Scientific Event-Henry Cavendish dicovered Hydorgen which he called "inflammable air".
Procedure
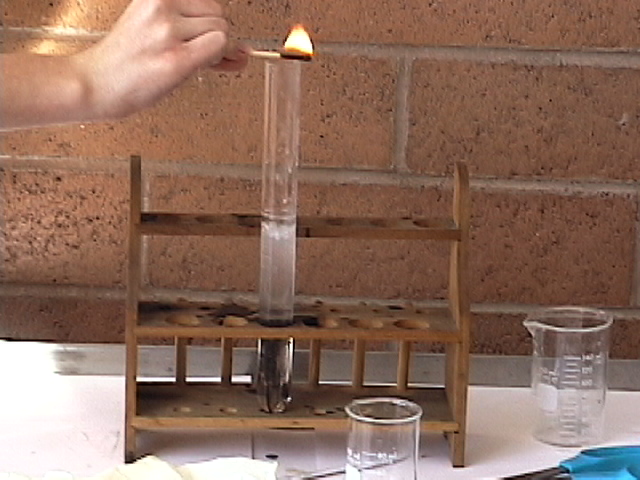
First fill the large test tube 1/4 of the way full of water. Second pour 30 mL sulfuric acid in to a beaker. Third step is to cut 2-3 inch strips of magnesium and place them into the the test tube of water. Fourth pour the sulfuric acid into the test tube; stir with a glass stirring rod so that the magnesium strips are completely covered. Let everything sit for ten to fifteen seconds so that the gas will build up. Fifth step is to light a match and hold it directly over the test tube. If it pops then the experiment has worked, if not then something was done wrong. Last of all, be careful because the material could be very hot.
Movie Of the Demonstration
Question:
1. How do I know a chemical reaction has taken place?
2. What is the product of combining Magnesium strips and Sulfuric acid?
The Final Balanced Chemical equation...